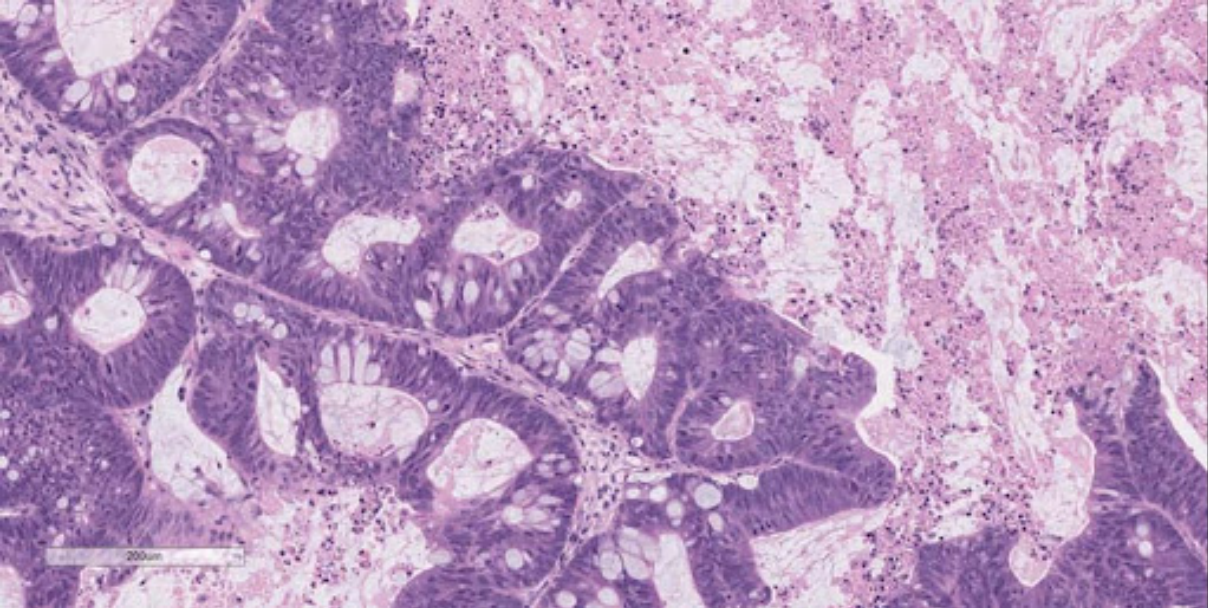
by Allen Badolian
With the war against COVID still raging on, and rightfully so, it is important to not
forget the original front lines, humanity’s perpetual strife with the deadliest of demons,
aka cancerous tumors. For decades, new developments and successes would only be
interrupted by failing drugs in clinical trials, and the continued presence of scientific
perplexity on the subject. Up until recently, it seemed like the battle was always one
step forwards and two steps back. Only in the last few months, groundbreaking
research is once again giving humanity the edge, in the form of the continued
development of Patient-Derived Xenografts (Figure 1).
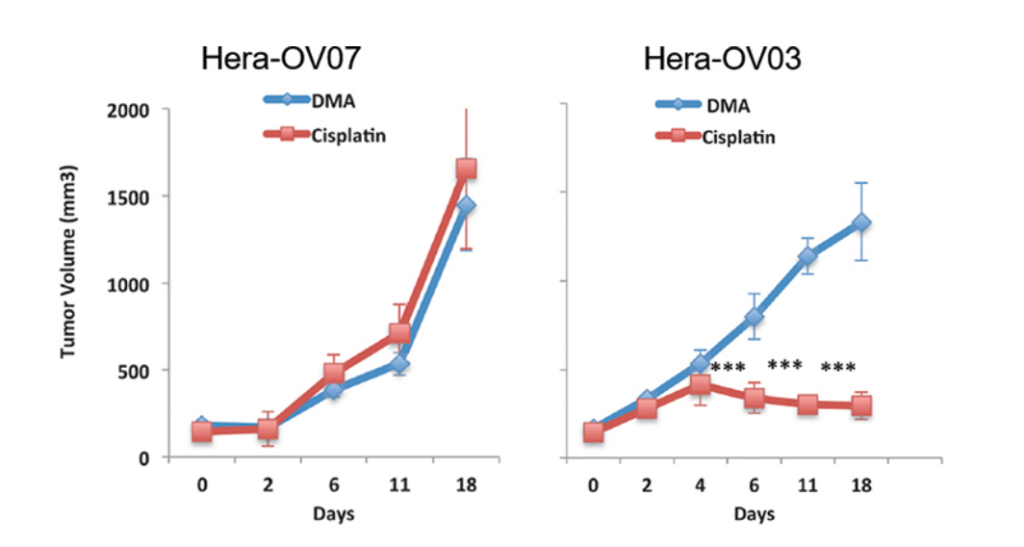
But we’ve heard this story before. A lack of true clinical-to-patient data once left
this technology destined to the limbo of so many before it. True drugs and treatments
are still few and far between, expensive, and often simply not good enough. We are
Figure 2: Assay measuring volume of two variants indicating strength of
transplant in hepatocellular carcinoma (Somasagara, 2021).
once again on the verge of something truly great. We need a new champion to propel
the revolutionary modeling of PDX into something we have never seen before, and
something revolutionary. Enter the simple freshwater minnow Zebrafish.
What is PDX?
PDX craze is sweeping the floor with competitive and successful tumor imaging.
Why? As has been pondered for decades, cancer is no simple condition. Think of it like
a computer virus. Once the host is “infected”, it becomes part of the environment, it
camouflages itself within the host’s delicate cellular communities. PDX allows for
transfer of tumors directly from targeted patient X into what is normally an
immunodeficient or SCID mouse. From here, potential treatment plans can be directly
monitored, and crossing into further generations can denote further effectiveness. I
believe that this is the single best way to go ahead with both phase I research trials as
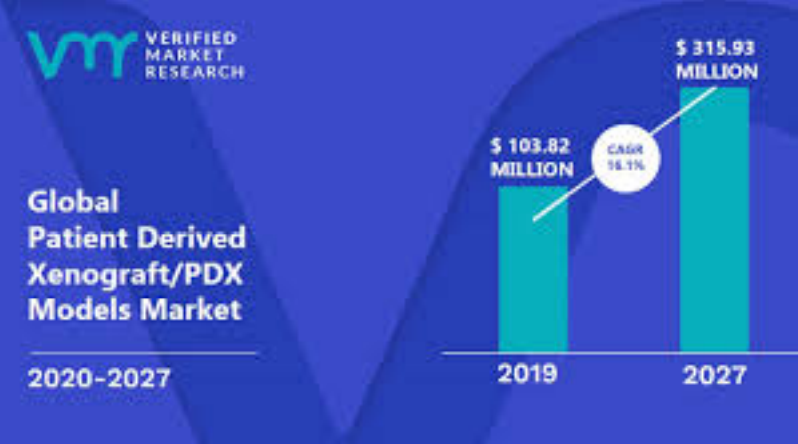
Figure 3: PDX market analysis (VTT research 2019)
well as patient-specific treatment plans. Cell line derived xenografts (CDX) of mice has
been the status quo, and the global PDX market based on prior research (-2019) is
expected to triple, however improvements on the technology are vital to success of
growth.
While CDX and knockout mice have been revolutionary, it is unfortunate that an
exponential amount of cancer treatments are either not fully developed or are much
less effective in human patients even when optimum conditions are attempted to be
replicated in the mouse model. This is because the mouse has fundamental
developmental intricacies that vary when compared to the human patient X. According
to the InternationalBiotech Innovation Organization there is a whopping 94.9% failure
rate for successful phase I to approval of cancer therapies. Additionally, I believe this is
too expensive and too unsuccessful. Figure 2 demonstrates how volume is not quite as
successful as it needs to be, with the volume dramatically shifting. How do we solve
this issue? Enter the zebrafish.
We know that the issue with Mice and the CDX is the developmental differences,
so to combat this, scientists went to the source. The zebrafish embryo, unlike any
vertebrates such as mice, is developed externally, this makes PDX much more widely
accessible as it bypasses the traditionally high costs of imaging. Additionally, zebrafish
have high fecundity, and are extremely rapid in their development. Does it work?
Researchers have targeted gastric cancer as a primary mission to utilize these
SCID zebrafish, and results have been overwhelming. 9 of 14 samples all showed
proliferating and transplanted modeling (including 5-fu sensitivity, specific to gc). Thus
the zebrafish PDX (known causally as zPDX) was confirmed as a reliable in vivo model
for gc.
Is everything perfect? Of course not. Tdp1 mediated chromosomal breaks which
are dispensable during larval developmental stages. This is contrasting with
hypersensitivity of TDP1- knockout vertebrate models. The lack of DNA breaks is
promising, but the DNA repairing factor that is required in vertebrates is not
demonstrated.There are these aspects of consistency which must be dully noted.
Personalized medicine is the key to solving this monumental crisis, and the true
solution is a team effort. The world must come united and researchers must combine
their efforts to demonstrate a clear plan utilizing PDX to solve case by case problems.
Researchers demonstrated the large scale screen capability of zebrafish models to
chronic myeloid leukemia involving SCID zebrafish transplantation. Thus it was proven
that ABL inhibition was discovered in vivo ABL inhibitor imatinib, MEK inhibitor U0126,
cytarabine, azacitidine and arsenic trioxide (Somasagara, 2021). Zebrafish were
examined histologically with drug treatment producing promising results. In vitro
analysis was also performed and fluorescently labeled cells were counted.
The results were significant. Research like this is fast and effective, truly leading
us to the front lines. Zebrafish PDX is a demonstration of how hard work and
determination can change the world.
Recourses
https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC5034890/
https://www.ncbi.nlm.nih.gov/pmc/articles/
PMC5034890/
https://www.cell.com/trends/cancer/fulltext/
S2405-8033(20)30121-7
What? Let us know in the comments.